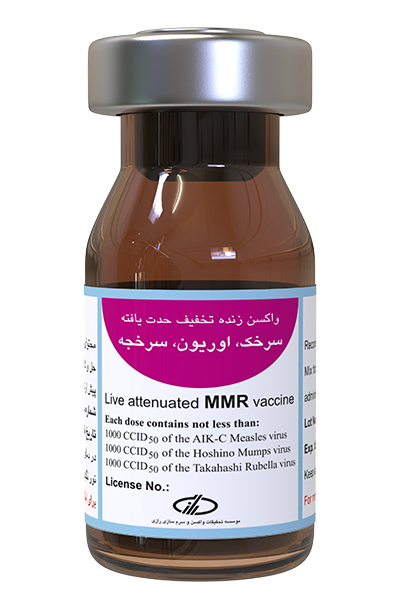
Type and form of vaccine:
Live, attenuated measles, mumps, and rubella (MMR) vaccine, freeze-dried
Vaccine compositions:
After preparation, each dose of the vaccine (0.5 ml) contains:
Active ingredients:
- At least 1000 (103 CC1D50) attenuated measles virus, AIK-C strain
- At least 1000 (103 CCID50) attenuated mumps virus, Hoshino strain
- At least 1000 (103 CC1D50) attenuated rubella virus, Takahashi strain
Non-active ingredients:
- Maximum 10 micrograms of kanamycin and neomycin
- Hydrolyzed gelatin, sorbitol, and sodium glutamate
- For replication of the virus used in this vaccine, human diploid cells (MRC-5) or chicken embryo fibroblasts are used.
Indication:
This vaccine is used for active immunization against measles, mumps, and rubella.
Administration route and dosage:
Before preparation, make the temperature of the solvent accompanying the vaccine (WFI) reach 2 to 8°C, then aseptically add it to the vial and shake gently until completely dissolved (without particles). Then inject 0.5 ml of the solution subcutaneously (preferably above the arm).
Recommended vaccination program:
Vaccination should be performed according to the National Immunization Committee's guidelines. For complete immunity, administration of two doses at 12 and 18 months of age is recommended. This vaccine may be used simultaneously with IPV, OPV, BCG, TT, dT, DTP, Haemophilus influenzae Type B, Hepatitis B, Yellow fever vaccines, and vitamin A complement.
Use during pregnancy and lactation:
Administering this vaccine to pregnant women (or women suspicious of being pregnant) is not permissible.
Contraindications:
- Sensitivity to any component of the vaccine (gelatin, neomycin,...)
- Children under 1 year of age, unless in special cases supervised by a physician
- Pregnant women (or women suspicious of being pregnant)
- High fevers or severe respiratory infections
- At least 3 months after the injection of blood, plasma, and immunoglobulins
- Radiotherapy, taking medication or illnesses that weaken the immune system
- Initial or acquired immunodeficiency, active untreated tuberculosis and malignancies
Side effects:
- After administering the vaccine, a mild fever approx. 37.8°C (5% of individuals experience fever over 39.4°C for 1-2 days or 7-12 days after vaccination), rashes (2% of individuals experience rashes for 2 days, 7-10 days after injection of the neck, the vaccine), itching, parotiditis, mild and temporary orchitis, hearing disorders, mild inflammation of lymph nodes behind temporary inflammation and pain in the joints, and in rare cases encephalitis and aseptic meningitis and thrombocytopenia are possible.
- A small swelling may appear in the injection area that will be absorbed and disappear gradually.
- In some cases, because of the gelatin in the vaccine, anaphylactic reactions may occur. In such cases, administering 1:10000 adrenaline, and other measures under the supervision of a physician are suggested.
Drug interactions:
- Do not use plasma and immunoglobulins for at least 3 months after administering this vaccine.
- Administering medication that weaken the immune system interfere with the use of this vaccine.
Precautions and warnings:
- In case of anaphylactic reactions, administering adrenaline under a physician's supervision is recommended.
- Because of the possibility of teratogenic effects, it is suggested to refrain from becoming pregnant for at least 3 months after administering the vaccine.
- Shake the vial containing vaccine gently before every injection until it is uniformed thoroughly.
- The prepared vaccine may be used until up to & hours, if cold chain and aseptic conditions are observed.
- Do not use vaccine after the expiration date.
- Prevent the prepared vaccine from freezing. If this happens, do not use vaccine.
- Consider aseptic conditions when injecting the vaccine.
Safe disposal of waste or partially used vaccines:
Single-use equipment used, and empty vaccine vials should be placed in the sharp-equipment container, and sterilized correctly (autoclave, burning, or using appropriate chemicals), and then buried hygienically.
Storage:
This vaccine should be stored and transported at 2 to 8°C and away from sunlight. In these conditions, it may be used until the expiration date written on the label. Storing the lyophilized vaccine below 0°C is permitted.
Packaging:
This vaccine is distributed in 5-dose and 2-dose lyophilized vials along with the solvent.
Last reviewed:
22.12.2018
References:
1- Immunization schedule and guidelines approved by the National Immunization Committee, 1394
2- Martindale, the complete drug reference, 39'h edition.
3- WHO Technical Report Series No. 840
 Related Products
Related Products Related News
Related News Related News
Related News Related Articles
Related Articles Live Attenuated MMR Vaccine (2, 5 doses)
Live Attenuated MMR Vaccine (2, 5 doses)