 Related Products
Related Products Related News
Related News Related News
Related News Related Articles
Related Articles Hexavalent Snake Antivenom Immunoglobulins
Hexavalent Snake Antivenom Immunoglobulins
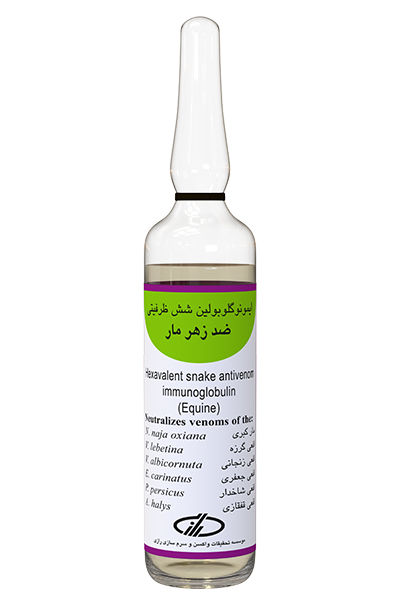
Type and form of product:
Purified immunoglobulin, injectable liquid
Product compositions:
This product is made from purifying and condensing of Hyperimmune horse plasma against 6 types of snakes’ venom as follows:
The active ingredients of this product include the fragment of immunoglobulins’ F(ab′)2. Each milliliter has the ability to neutralize more than 50 LD50 of the venom of each of the mentioned snakes based on its lethal property on the mice.
This product contains up to 0.25% phenol as a preservative.
Indication:
For neutralizing the snakes’ venom mentioned above.
Administration route and dosage:
Since the amount of received venom among the bitten children or adults is the same, they should be taken the same amount of antivenom, regardless of their ages and heights. However, children and weak people are at high risk. Considering the severity of the symptoms and clinical complications of the bitten person, it is usually recommended to administer 1 to 2 ampoules via intravenous infusion, as an initial dose, to neutralize the venom. In severe cases, especially if there is a delay in treatment, administration of more ampoules will be required. This amount depends on the results of the tests and the clinical examination and is prescribed by the physician.
In case of persistence or recurrence of bleeding, as well as the persistence or worsening of neurological and cardiovascular symptoms: repeat the initial dose after 1-2 hours.
In case of persistence or reversal of coagulation problems: Repeat the initial dose after 6 hours
Appropriate time and how to use:
This product should be prescribed immediately after the snake bite via intravenous infusion (It should be diluted with 250-500 ml of 9% saline or 5% glucose solution. Infusion speed should be 250 ml/hr). If there is no necessary facility for infusion, antivenom could be injected intravenously by using of a syringe and the rate of 2 ml/min, under supervision of a physician.
How to use during pregnancy and lactation:
There is no information about Undesirable effect of this product during pregnancy and lactation.
Contraindications:
Side effects:
Due to the fact that this product is made from horse plasma derivatives and is a heterologous agent for humans, it is possible to observe the following side effects:
Note: Individuals who receive antihistamine or corticosteroid are less likely to face with late side effects.
Therapeutic measures in case of occurrence of anaphylactic shock:
Drug interactions:
There has been not a comprehensive study about the drug interactions of this product yet.
Precautions and warnings:
Notices:
Safe disposal of waste or partially used ampoules:
Empty or partially used ampoules must be properly sterilized by autoclaving, burning or appropriate chemicals.
Storage
Keep the antivenom at 2 to 8 °C away from light. In this condition, it can be used up to the expiry date stated on the product label.
Packaging:
This product is provided in single box, containing 10 milliliters ampule.
Last reviewed:
10.2018
References:
1-David A. Warrel, 2010, guidelines for the management of snake-bites, world health organization- regional office for south-east Asia.
2- WHO Expert Committee on Biological Standardization, 2017, Guidelines for the production, control and regulation of snake antivenom immunoglobulins, TRS, No. 1004, annex 5